28 Jun 2024
Research blog: Immunotherapy for blood cancers – the rise of CAR-T cells
Immunotherapy has revolutionised the treatment landscape for various cancers, including blood cancers such as leukaemia, lymphoma, and multiple myeloma. Though immunotherapy has been used since the 1950s(i) with the introduction of bone marrow transplants, more targeted cancer treatments utilising immunotherapy have gained a footing since the 1990s.(ii) This approach works by harnessing and boosting the body’s own immune cells to destroy cancer cells. Specific methods include: monoclonal antibodies, donor lymphocyte infusion, interferon, and CAR-T cell therapy.
In many cases, immunotherapy is used when leukaemia has returned or relapsed after treatment or when other treatments, such as chemotherapy, have not been effective. Because it does not kill healthy cells, there are fewer side effects associated with immunotherapy. For some types of leukaemia, immunotherapies are incorporated into the initial treatment plan, and often combined with other treatments such as chemotherapy or targeted therapy. For instance, the monoclonal antibody rituximab is used in treating chronic lymphocytic leukaemia (CLL).(iii)
Among these emerging therapies, CAR-T cell therapy has drawn significant attention. While it offers notable benefits, it’s crucial to consider its limitations and challenges. In this article, we delve into CAR-T therapy and shed some light on this rising star.
What is CAR-T cell therapy?
Chimeric Antigen Receptor T-cell (CAR-T) therapy is a type of immunotherapy that involves modifying a patient’s T-cells, a type of immune cell, to better recognise and attack cancer cells. The process begins with the extraction of T-cells from the patient, which are then genetically engineered in a laboratory to express chimeric antigen receptors. These are specially designed proteins that help T-cells to find and attack cancer cells. The modified T-cells are then multiplied and infused back into the patient, where they seek out and destroy cancer cells. As these are the patient’s own cells, the possibility of rejection is significantly decreased, and the cells only target cancer cells, further reducing the chance of rejection.
As this remains a relatively novel approach, there are currently only two approved indications for the use of CAR-T in the UK across 17 centres. The NHS provides CAR-T therapies for children and young people with B cell acute lymphoblastic leukaemia, and NICE has recommended CAR-T therapy for adults with diffuse large B-cell lymphoma and primary mediastinal B-cell lymphoma in England. More information on the treatment availability can be found on the NHS website.
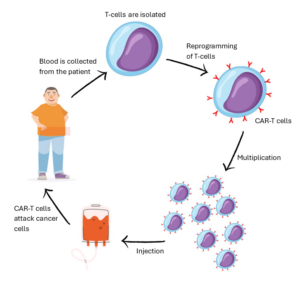
Illustration of how CAR-T cells are made
Figure 1: showing an illustration of how CAR-T cells are made. First a sample of blood is collected from the patient, the T-cells (a white blood cell) are isolated and reprogrammed to recognise a particular antigen, forming a CAR-T cell. These are then multiplied and then infused back into the patient where they are left to find and attack cancer cells.
The challenges and limitations of CAR-T therapy
While CAR-T therapy is a ground-breaking advancement in cancer treatment, it comes with significant risks. One major concern is severe side effects, such as cytokine release syndrome (CRS) and neurotoxicity. CRS, an intense immune response, can cause high fever and organ failure, while neurotoxicity involves damage to the brain or nervous system. Managing these side effects requires specialised care, which can burden healthcare systems. Additionally, early studies suggest that CAR-T therapy may carry a cancer risk itself.(iv/vi)
Obstacles to the widespread use
Several challenges impede the widespread use of CAR-T therapy. CAR-T cells can lose effectiveness over time due to exhaustion and aging. Isolating enough high-quality T-cells from patients is another common problem. The entire process of harvesting, modifying, and replicating the cells is time-consuming and requires specialised equipment and trained personnel, leading to substantial costs. Furthermore, the risk of immune rejection, similar to organ transplantation, limits its clinical use.
Despite these challenges, the promise of CAR-T therapy continues to drive research and innovation, aiming to overcome these barriers and make this revolutionary treatment more accessible and effective for patients worldwide.
Improvements in the CAR-T landscape and the emerging role of CRISPR
One way to make CAR-T therapy more available is by creating an ‘off-the-shelf’ version. Currently, the process is individualised for each patient. However, research is focused on making the process more efficient and potentially building a stock of CAR-T cells for multiple patients. A technique showing promise in this field of immunotherapy is CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats). Although still experimental, the technology is emerging as a potential game-changer.
CRISPR allows for precise editing of DNA, which could make cancer treatment even safer and more effective. Researchers are exploring ways to use CRISPR to improve CAR-T cell therapy by making T-cells more effective and less likely to cause severe side effects. This could be used not just to make more precise CAR-T cells but also to eliminate the genes that trigger immune rejection. These engineered cells could then be mass-produced and frozen, ready to be distributed in hospitals. This eliminates the need to harvest, edit, and expand cells individually for each patient, streamlining treatment and reducing logistical complexities.
Conclusion
CAR-T is a brilliant demonstration of how research can continuously strive for more effective, safer, and accessible treatment options. Chemotherapy was once a ground-breaking discovery that saved thousands of lives, but the quest for better treatments never ceases. As technologies advance, the hope for more precise and efficient treatments grows, promising a future where the limitations of current therapies are addressed and overcome. Balancing optimism with a realistic understanding of the challenges ahead remains essential as we navigate this rapidly evolving field.
Discover our research blogs.
References:
(i) – https://cancer.umn.edu/mncctn/news/blood-cancer-awareness-history-bone-marrow-transplantation Accessed 19/06/2024
(ii) – Dobosz P, Dzieciątkowski T. The Intriguing History of Cancer Immunotherapy. Front Immunol. 2019 Dec 17;10:2965. doi: 10.3389/fimmu.2019.02965. PMID: 31921205; PMCID: PMC6928196.
(iii) – Isidori A, Cerchione C, Daver N, DiNardo C, Garcia-Manero G, Konopleva M, Jabbour E, Ravandi F, Kadia T, Burguera AF, Romano A, Loscocco F, Visani G, Martinelli G, Kantarjian H, Curti A. Immunotherapy in Acute Myeloid Leukemia: Where We Stand. Front Oncol. 2021 May 10;11:656218. doi: 10.3389/fonc.2021.656218. PMID: 34041025; PMCID: PMC8143531.
(iv) – Zhang Y, Qin D, Shou AC, Liu Y, Wang Y, Zhou L. Exploring CAR-T Cell Therapy Side Effects: Mechanisms and Management Strategies. J Clin Med. 2023 Sep 22;12(19):6124. doi: 10.3390/jcm12196124. PMID: 37834768; PMCID: PMC10573998.
(vi) – https://www.nature.com/articles/d41586-024-01215-0 Accessed 03/06/202
Related posts
17 September 2024
The food industry’s great and good come together to celebrate 25 years of Who’s Cooking Dinner? and raise over £280,000 for leukaemia research.
London’s hottest charity culinary event, Who’s Cooking Dinner?, celebrated its 25th anniversary at The Dorchester on Monday (16th September). It was an event to remember with chefs including Tom Kerridge,…
4 September 2022
Worrying numbers of leukaemia patients are being misdiagnosed or waiting too long for a blood test, say leading UK leukaemia charities
Patients in the UK face “the luck of the draw” when presenting with leukaemia symptoms. GPs are often left without adequate support to provide blood tests or process the results…
19 June 2023
Leukaemia UK research paves the way for personalised lymphoma treatment
Despite promising trials, standard treatment for diffuse large B-cell lymphoma (DLBCL) hasn’t changed in a decade. New treatment strategies for this type of blood cancer are urgently needed. Could the…
30 November 2023
Leukaemia UK invests in next generation of blood cancer trial leaders
This week, aspiring chief investigators of future cutting-edge blood cancer clinical trials took to Birmingham for the DIDACT Foundation’s inaugural Clinical Trials Workshop – an event funded by Leukaemia UK….